
Danielle Tan
Managing Consultant
” When it comes to HACCP validation, verification and monitoring, wrapping our minds around it is crucial and not always easy.”
– 5 mins read

In the Codex HACCP 2020 standard, there is a guidance on 3.9 Establish a Monitoring System for Each CCP and 3.11 for Validation of the HACCP Plan and Verification Procedures:
Clause 3.9 Establish a Monitoring System for Each CCP
Monitoring of CCPs is the scheduled measurement or observation at a CCP relative to its critical limits. The monitoring procedures should be able to detect a deviation at the CCP. Further, the monitoring method and frequency should be capable of timely detection of any failure to remain within critical limits, to allow timely isolation and evaluation of the product. Where possible, process adjustments should be made when monitoring results indicate a trend towards a deviation at a CCP. The adjustments should be taken before a deviation occurs.
Clause 3.11.1 Validation of the HACCP Plan
Before the HACCP plan can be implemented, its validation is needed; this consists of making sure that the HACCP elements together are capable of ensuring control of the significant hazards relevant to the food business: identifying the hazards, critical control points, critical limits, control measures, frequency and type of monitoring of CCPs, corrective actions, frequency and type of verification and the type of information to be recorded.
Clause 3.11.2 Verification Procedures
After the HACCP system has been implemented, procedures should be established to confirm that the HACCP system is working effectively. These include procedures to verify that the HACCP plan is being followed and controlling hazards on an ongoing basis, as well as procedures that show the control measures are effectively controlling the hazards as intended. Verification also includes reviewing the adequacy of the HACCP system periodically and, as appropriate, when changes occur.

So, what exactly do we mean when we say “Validation”, “Verification” and “Monitoring”?
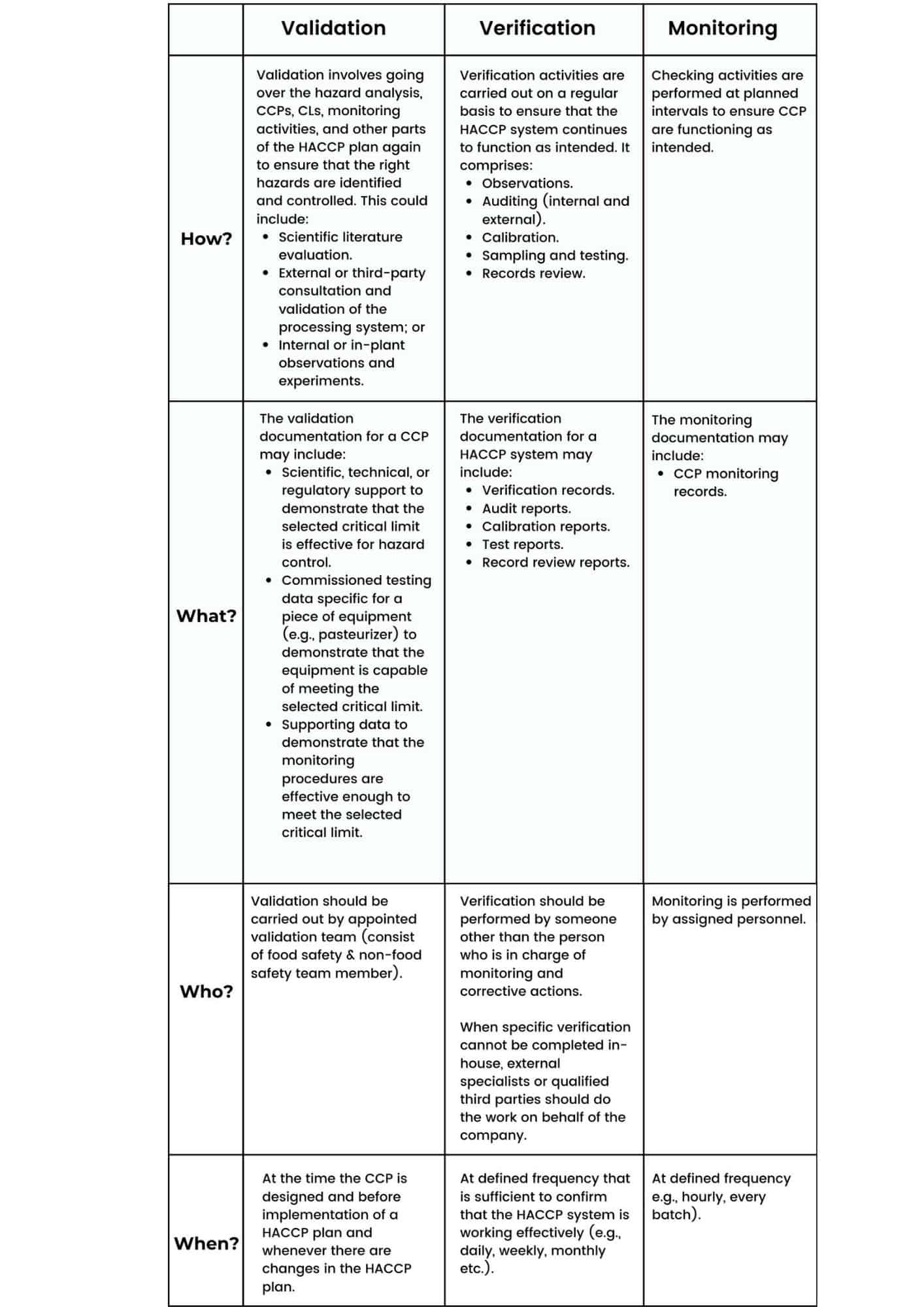
Validation is the process of obtaining evidence that the elements of the HACCP plan (CCP critical limits) are effective.
Verification is the process of checking using defined methods, procedures, tests and other evaluations, in addition to monitoring, to determine compliance with the HACCP plan.
Monitoring is a routine activity which provides evidence that a CCP is operating as intended. It is not verification.
So, in simple words:
Validation is – proving that it will work.
Verification is – proving that it is working according to the plan.
Monitoring is – proving that it is being done according to the plan.
The Differences between Validation, Verification and Monitoring:
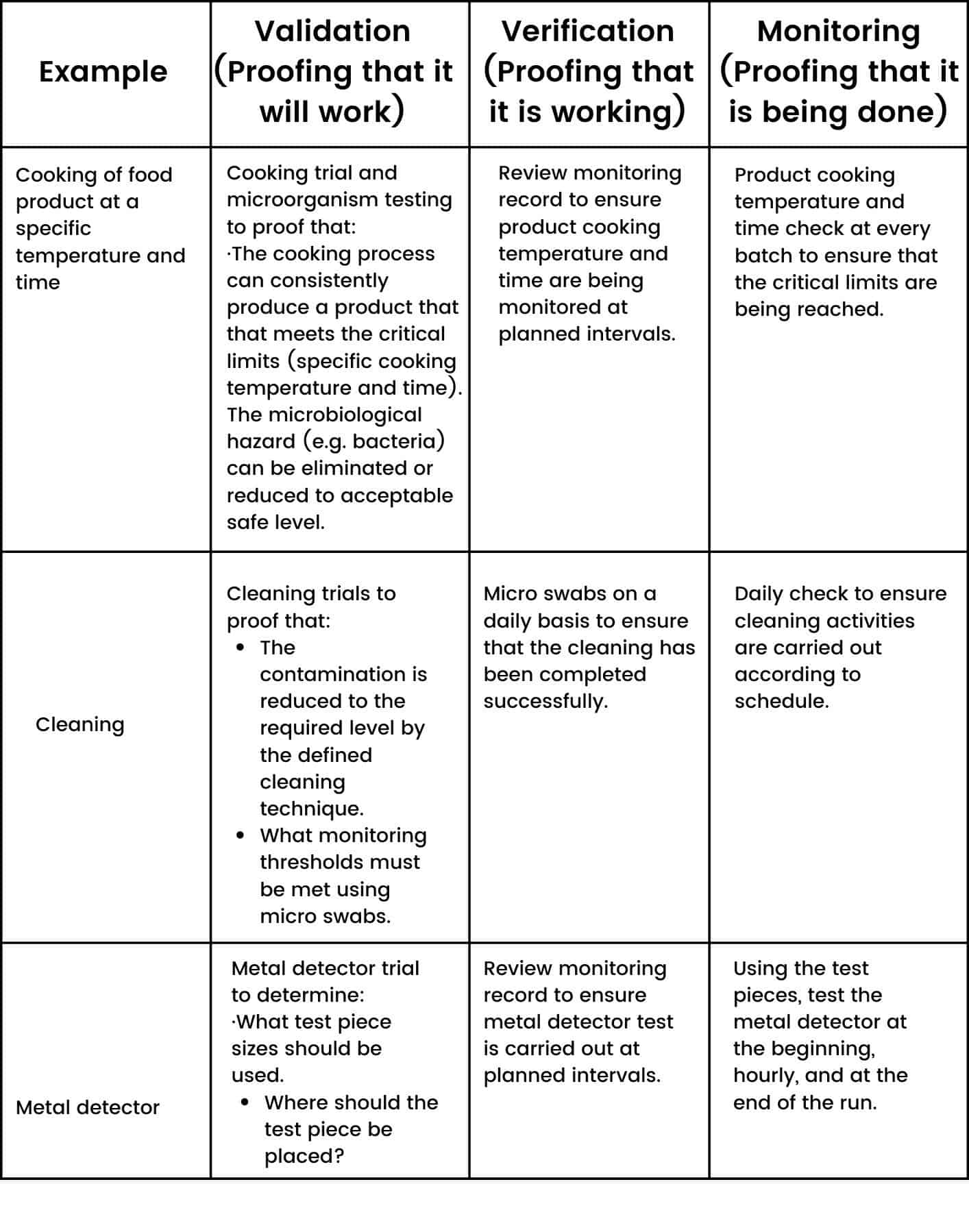
Here are some validation, verification and monitoring examples:
Review Your HACCP Plan Regularly
And finally, don’t forget about your HACCP plan once you’ve completed it. The program is a live, dynamic document and plan that should be reviewed and revised on a regular basis to reflect the facility’s, customers’, and regulators’ changing needs.
Reference :
- Codex Alimentarius General Principles of Food Hygiene CXC-1 1969 (version 2020)
- https://techni-k.co.uk/uncategorised/haccp-validation-verification/
- https://safefoodalliance.com/haccp/the-6th-haccp-principle-verification/
More Article
FSSC 22000 Version 7 Update: What Food Businesses Should Prepare Before Transition
How Food Businesses Should Plan for the Upcoming Transition The Foundation FSSC has indicated that FSSC 22000 Version 7 is expected to be published in May 2026, followed by an approximately 12-month transition period for certified organisations and certification...
ISO 9001:2026 Revision Update: What Organisations Should Prepare Before Publication
Preparing for the Upcoming ISO 9001 Revision ISO 9001, the international standard for Quality Management Systems (QMS), is currently undergoing revision, with the updated edition ISO 9001:2026 expected to be published in the second half of 2026, replacing ISO...
ISO 14001:2026 Revision: Key Changes Expected in Environmental Management Systems
What Organisations Should Prepare Before the Expected 2026 Publication ISO 14001, the international standard for Environmental Management Systems (EMS), is currently undergoing revision. The updated edition ISO 14001:2026 is expected to be published around April 2026,...
CIDB ISO 37001 Requirement for G7 Contractors
Source: CIDB Malaysia announcement on mandatory ISO 37001 Anti-Bribery Management System certification for G7 contractors effective January 2027. What Construction Companies Should Prepare Before 2027 CIDB Malaysia has announced that ISO 37001 Anti-Bribery Management...









